Overview
Background: In previous years, management of eosinophilic oesophagitis (EoE) in the UK has been largely arbitrary due to the lack of clinical guidelines.
What’s new?: The EoE Guidelines Development Group of the BSG’s Oesophageal Section have reviewed available literature and formulated the UK’s first BSG and BSPGHAN clinical guidelines on the diagnosis and management of eosinophilic oesophagitis.
Clinical Impact: Diagnostic pathways and treatment algorithms for eosinophilic oesophagitis.
Introduction
Eosinophilic oesophagitis (EoE) is the most common of cause of food bolus obstruction in patients presenting to the emergency department. It is also a common cause of dysphagia in adults, and it is associated with retrosternal discomfort, and non-cardiac chest pain.
The prevalence of EoE in adults in the Western population is rising (≈ 63/100,000).
EoE is more common in males, Caucasians, and patients with atopy. Symptoms may have seasonal variation.
EoE can occur on its own or co-exist with gastro-oesophageal reflux disease (GORD).
Diagnosis
EoE is an immune-allergic inflammatory disease of the oesophagus defined by symptoms of dysphagia and/or food impaction in adults, with a peak eosinophile count ≥ 15 eosinophils/high power field, or ≥15 eosinophils/0.3 mm2, or >60 eosinophils/mm2.
Besides the peak eosinophil count, other histological features of EoE should also be present. These include the presence of basal cell hyperplasia, oedema (spongiosis), eosinophil microabscesses, eosinophil layering, eosinophil degranulation and subepithelial sclerosis.
Have a high index of suspicion in patients presenting with food bolus obstruction.
*** ≥ 6 oesophageal biopsies from at least two different anatomical sites are recommended. Note: should be off proton pump inhibitors (PPIs) for 3 weeks for an accurate diagnosis.
Consider other causes of eosinophilia or partially treated EoE if biopsies show < 15 eosinophils/0.3 mm2.
Other causes of oesophageal eosinophilia include GORD, achalasia, infection (fungal or viral), Pill oesophagus, hypereosinophilic syndrome, drug hypersensitivity reactions, coeliac disease and connective tissue diseases.
A diagnostic algorithm has been proposed:
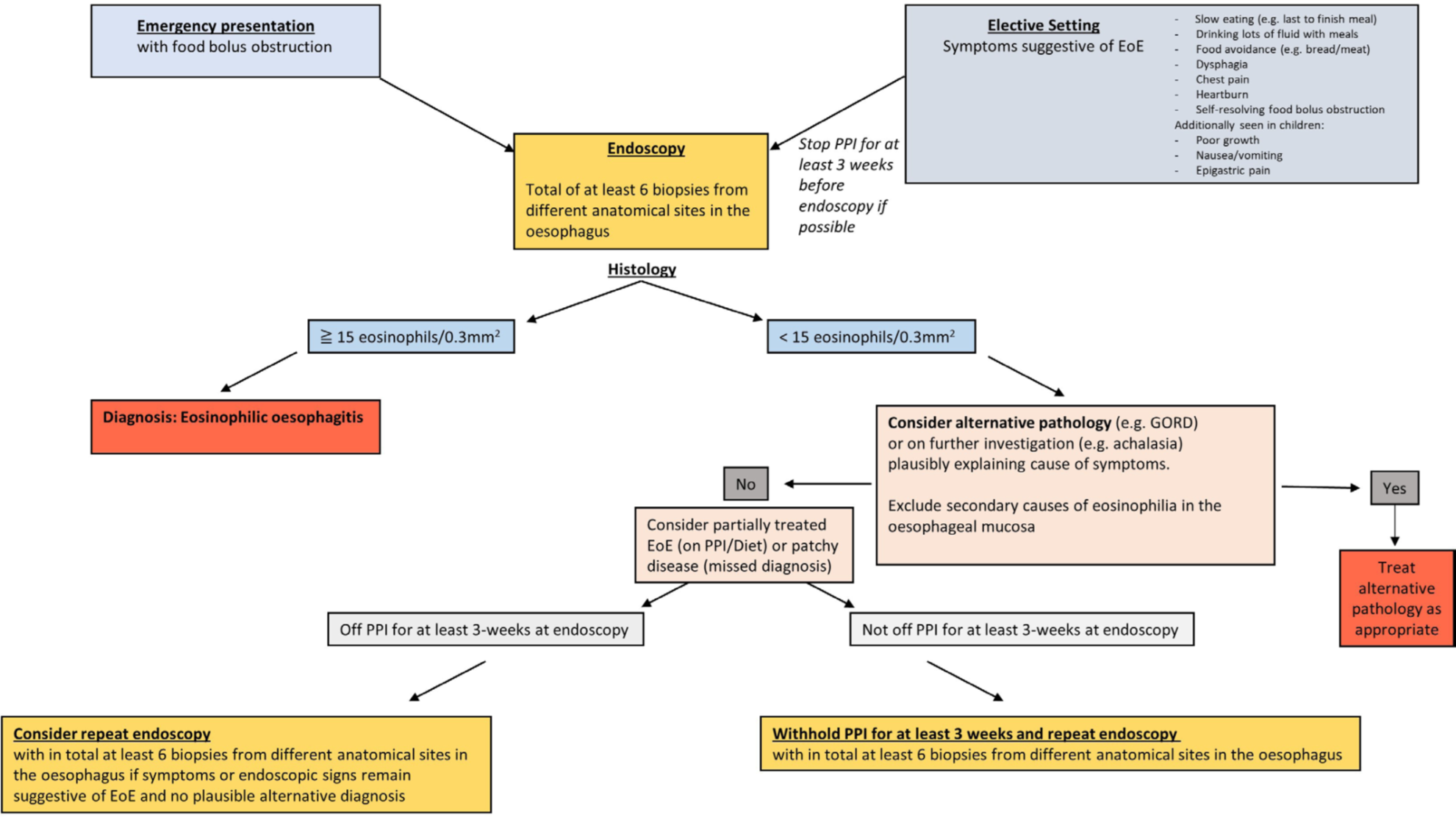
Image adapted from Dhar A et al (see reference, figure 2).
Medical Management
First line
Dietary (recommended in only selected patients) or Drug intervention (preferred)
Diet: 2 food/4 food/6 food exclusion diets. Escalate diet every 8-12 weeks if no or partial response. Referral to a dietician is recommended.
PPIs (e.g. Omeprazole 20mg BD) OR topical steroids (e.g. Oro-dispersible Budesonide 1mg BD for induction of remission, then either 1mg BD or 0.5mg BD for maintenance of remission).
Second line
Combination therapy for select/difficult cases + specialist dietician input i.e.
PPIs (e.g. Omeprazole 20mg BD) AND steroids (e.g. Budesonide 1mg BD) + dietician
Consider specific mono-clonal antibody (in co-existing severe allergic disease), clinical trials.
Endoscopic Management
Consider oesophageal balloon dilatation if stricturing disease is present, together with Oro-dispersible Budesonide.
Repeat biopsies 8-12 weeks after the initiation of treatment is recommended to check for remission. There is only a moderate correlation between symptoms and histological remission.
No consensus on the frequency of follow up endoscopies and clinic follow up at present.
Histological remission is defined as < 15 eosinophils/0.3 mm2 (can consider de-escalating medication/diet)
Oesophageal physiological testing should be considered in patients with eosinophilic oesophagitis who have ongoing dysphagia despite histological remission and the absence of fibrostenotic disease at endoscopy.
A management algorithm has been proposed:
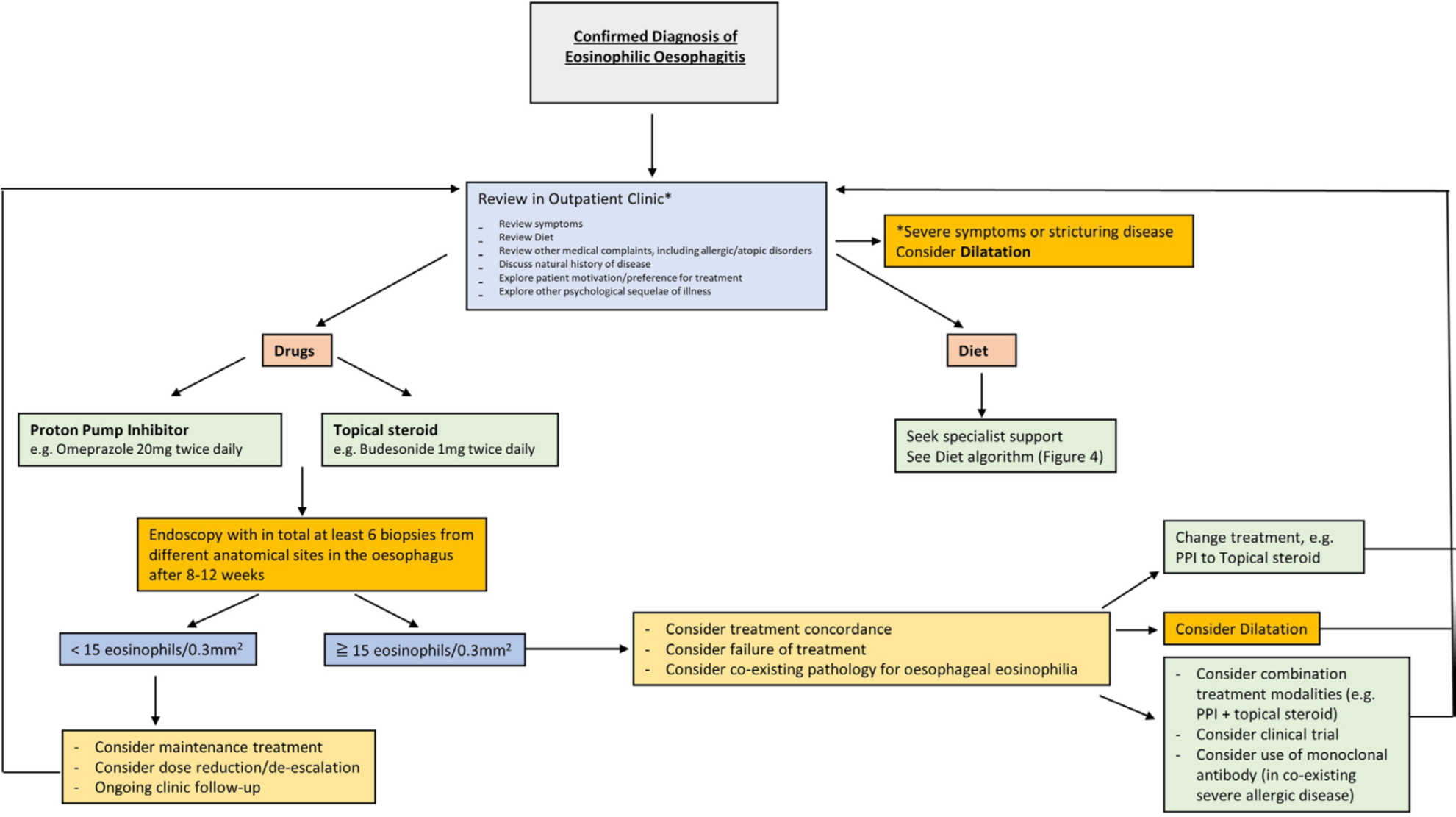
Image adapted from Dhar A et al (see reference, figure 3).
Useful tips
Instead of swallowing corticosteroid puffs from Budesonide or Fluticasone inhalers, Jorveza ® (topical budesonide) is now available in the BNF formulary and is licensed for use in the treatment of EoE. Dose of Jorveza® = 1mg BD for 6-12 weeks
Dupilumab (Dupixent), a interleukin 4 and interleukin 13 monoclonal antibody, has recently been approved by the FDA (USA) for use in the treatment of EoE. Dupilumab is currently available in the BNF for use in moderate-to-severe atopic eczema, severe asthma with type 2 inflammation, and severe chronic rhinosinusitis with nasal polyps. Dupilumab may be licensed for the treatment of EoE in the UK in the near future.
This article is mapped to the following JRCPTB learning objectives:
- Knows the various causes of dysphagia and their clinical presentations
- Understands the methods of assessment and investigation including the use of manometric assessment where appropriate
- Knows the range of therapeutic options including the potential for endoscopic treatment, and how to select appropriate treatment
Please email comments or suggestions to Dr John Ong [email protected], section editor.
Author Biography
Prof. Anjan Dhar has an interest in luminal gastroenterology and has been trained in inflammatory bowel disease and advanced therapeutic endoscopy and colonoscopy in India and subsequently at the University of Oxford and University College London. He has held the British Society of Gastroenterology International Fellowships at the Medical University of South Carolina USA and Tokyo Japan for early GI cancer diagnosis and endoscopic treatment.
Q&A
-
e. The eosinophil count of 8 eosinophils/0.3 mm2 is not diagnostic of eosinophilic oesophagitis. In the presence of signs and symptoms of eosinophilic oesophagitis, an eosinophil count < 15 eosinophils/0.3 mm2 may represent partially treated eosinophilic oesophagitis. There is no indication to start oral budesonide or double the dose of omeprazole with the clinical information given. Oesophageal eosinophilia may be associated with achalasia (see reference, table 1).
-
a. Positive Warthin-Starry staining is usually associated with Helicobacter pylori, spirochetes, or microsperediates infection.
CME
Quality Indicators in Barrett's Endoscopy: A Quest for the Holy Grail
27 October 2025
Factors associated with upper GI cancer occurrence after endoscopy
24 February 2025
Masterclass: Achalasia - Beyond the Basics
05 November 2024
Dhar A, Haboubi HN, Attwood SE, et al. Gut 2022. Epub ahead of print: doi:10.1136/gutjnl-2022-327326
