Authors:
Dr Alvin Ochieng, Consultant Gastroenterologist, and Dr Adler Ma (ST7)
Name of teams involved:
Dr Alvin Ochieng, Dr Roxane Kiu Yan Lam (FY1), Dr Tun Pauk (IMT1), Dr Adler Ma (ST7) and Dr Merella Al Tali (FY1)
Acknowledgement:
Infection prevention and control nurses: Debra Charles, Sally Casey, Julie Marriott, Sue Dixon, George Wallis and Sneha John
Specialist Pharmacist Antimicrobials: Dr Caroline Hallam
IP&C Doctor: Dr Catherine Tremelett
IP&C - administrator: Ellie Johnson
FMT team: Prof Arjan Narbad and Dr Elumogo Ngozi
Challenges to the service and the need for change
Clostridioides difficile is the primary cause of infectious diarrhoea among hospitalised patients, necessitating prompt initiation and appropriate escalation of treatment to avert life-threatening complications. According to the latest guidelines from NICE (NG199), vancomycin is now the first-line antimicrobial therapy. Fidaxomicin serves as the second-line treatment when vancomycin proves ineffective and is the preferred option for treating relapses occurring within 12 weeks. Faecal Microbiota Transplantation (FMT) is an option for refractory C. Difficile cases. NICE also emphasises the importance of a multidisciplinary approach for hospitalised patients with C. difficile infection, involving a microbiologist, pharmacist, and infection prevention and control (IPC) nurse, as practised in our trust.
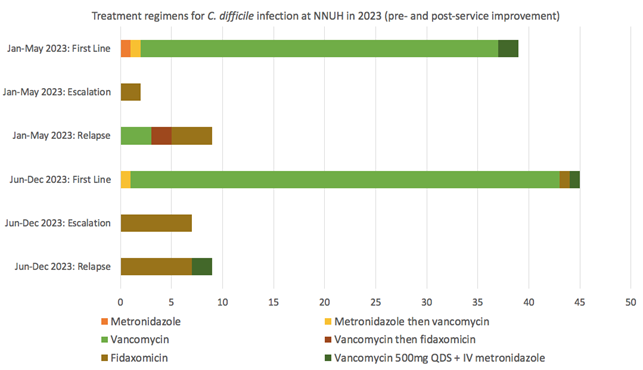
From January to May 2023, our hospital treated 39 patients with active C. difficile infections primarily with vancomycin. In specific cases, treatments were adjusted to include high-dose vancomycin with intravenous metronidazole for severe colitis, or a combination of oral metronidazole and vancomycin. Only two patients were escalated to fidaxomicin following non-response to the initial treatment. Within 12 weeks, 9 cases (23%) relapsed; treatment protocols varied, including initial or subsequent use of fidaxomicin and vancomycin.
This period highlighted several challenges, including a significant number of relapses and a tendency to treat relapsing infections with vancomycin over fidaxomicin. Additionally, two instances of initial metronidazole monotherapy contrary to guidelines were noted, with one patient eventually relapsing after completing metronidazole treatment without switching to vancomycin. These prescribing errors were attributed to the adjustment period required for familiarising with the updated NICE guidelines.
The process of securing approval for fidaxomicin as a second-line treatment faced delays, only receiving endorsement from our local Drug and Therapeutics Committee in spring 2022, despite NICE's recommendation in July 2021. Our hospital previously offered a FMT service which was discontinued due to unavoidable circumstances. The absence of this service presented additional challenges in managing refractory C. difficile cases and who require FMT as a treatment.
How the challenges were addressed
Since June 2023, patients at our trust with C. difficile infection have been evaluated at a multidisciplinary ward round including a consultant gastroenterologist, microbiologist, pharmacist and IPC nurse.
Our ward rounds consistently emphasise the importance of selecting the most effective therapy for our patients, significantly raising awareness. The multidisciplinary team (MDT) is committed to ensuring that all patients receive the recommended first-line treatment with vancomycin and promptly considers treatment escalation for those with a severity score of 3 or higher. Fidaxomicin as a second-line therapy following vancomycin failure or a regimen of high-dose vancomycin (500mg four times daily) combined with intravenous metronidazole for severe colitis.
The EXTEND trial of 2018 demonstrated that patients treated with extended-pulsed fidaxomicin experienced superior rates of sustained clinical cure at 30 days compared to those treated with vancomycin. Leveraging these findings, our MDT swiftly obtained approval from the Drug and Therapeutics and Medicines Committee to use extended-pulsed fidaxomicin. This approval process was notably more efficient than previous attempts to secure authorisation for second-line therapy i.e Fidaxomicin.
Furthermore, our expertise has enabled the re-development of an FMT pathway, although we have encountered new regulatory challenges. In response, our MDT has worked closely with the microbiology team at St Thomas’ Hospital in London to identify high-risk patients for FMT referral as a temporary solution until we can launch and license our own FMT service for patients in East Anglia. We have now established an FMT clinic and created consent forms for patients undergoing FMT, ensuring a structured and patient-centred approach to this innovative treatment option.
Outcomes of service improvement
From June to December 2023, 45 patients were offered first-line treatment. This consisted of vancomycin alone in 41 cases, high-dose vancomycin with intravenous metronidazole in one case with severe colitis, oral metronidazole followed by vancomycin in one case, and fidaxomicin in one case. 7 patients (15.5%) were escalated to fidaxomicin due to suboptimal response to first-line treatment. There were 9 confirmed cases (20%) of relapse within 12 weeks: 7 treated with fidaxomicin and 2 treated with a combination of high-dose vancomycin plus intravenous metronidazole due to fulminant colitis.
Following the introduction of the C. difficile MDT, all patients with C. difficile relapse were appropriately treated with either fidaxomicin or high-dose vancomycin plus intravenous metronidazole (for severe colitis). Furthermore, a considerably higher number of patients (15.5% vs 5.1%) underwent treatment escalation to fidaxomicin during their index infection. Overall rates of relapse were similar (20% vs 23%) but disease severity and patient comorbidity must be taken into account when comparing these figures.
Three patients were escalated to third-line therapy with extended pulsed fidaxomicin after relapsing despite treatment with fidaxomicin. Two patients had sustained cure following extended pulsed fidaxomicin. One died before completing the course of the treatment.
Not included in the above figures, two patients with refractory C. difficile were referred for and received FMT following review at a dedicated FMT clinic and discussion within the multidisciplinary team.
Learning points
The findings here indicate that our multidisciplinary model of managing patients with C. difficile is effective at ensuring early treatment escalation for non-responders, appropriate antimicrobial selection for relapses within 12 weeks, and provision of third-line strategies for refractory and complex cases.
Our service improvement strategy involved close collaboration between the gastroenterology, microbiology, pharmacy and nursing teams. Our microbiologists have continued to enforce the escalation process for antimicrobial selection in line with evidence-based guidelines, whilst gastroenterology input has contributed to justifying treatment decisions from a clinical perspective, particularly with respect to third-line strategies. We have moved away from a ‘one size fits all’ process and towards more individualised approaches to treating C. difficile infections based on severity scores and risk of relapse. Working together, we have secured approval for and successfully introduced third-line strategies for refractory infections. Pathways have been established for the management of complex cases and we are now building on our experience to set up an FMT service for the East Anglia region.
Read More

