Authors: Melanie Cuffe1, Nigel Scott2, Clare Donnellan1
1Leeds Gastroenterology Institute, St James’s University Hospital, Bexley Wing, Beckett Street, Leeds LS9 7TF, UK
2Department of Histopathology, St James’s University Hospital, Bexley Wing, Beckett Street, Leeds LS9 7TF, UK
Case
A 65-year-old man presented with abdominal pain and more than 10 episodes per day of watery stool with blood and mucus (Common Terminology Criteria for Adverse Events [CTCAE] grade 3 diarrhoea; Table 1).1 He had received three cycles of chemotherapy and immunotherapy (pembrolizumab) for metastatic lung adenocarcinoma and had a background of type 2 diabetes mellitus.
| Diarrhoea | Colitis | |
| Grade 1 | 3 times baseline | Mild changes, no symptoms |
| Grade 2 | 4‒6 times baseline | Pain, blood/mucus per rectum |
| Grade 3 | >7 times baseline | Severe pain, ileus, peritonism, fever |
| Grade 4 | Life-threatening consequences | Life-threatening consequences |
| Grade 5 | Death | Death |
Table 1: Common Terminology Criteria for Adverse Events after cancer therapies (CTCAE - National Cancer Institute)
Question 1
Computerised tomography (CT) suggested uncomplicated acute sigmoid diverticulitis, as well as reduction in tumour size. Flexible sigmoidoscopy demonstrated active proctocolitis, which involved areas without diverticula, refuting the diagnosis of diverticulitis and suggesting immunotherapy-induced colitis. The latter diagnosis was confirmed histologically. Stool cultures were negative for all bacteria (including Clostridium difficile) and viruses.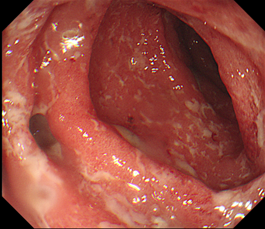
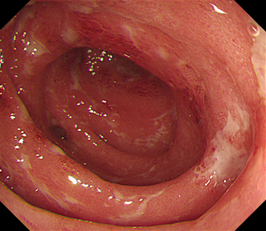
Figure 1: Endoscopic images demonstrating widespread superficial ulceration with diverticulosis
A
B
C
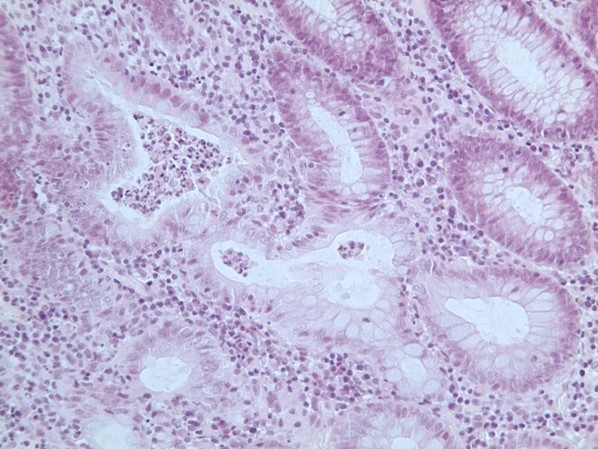
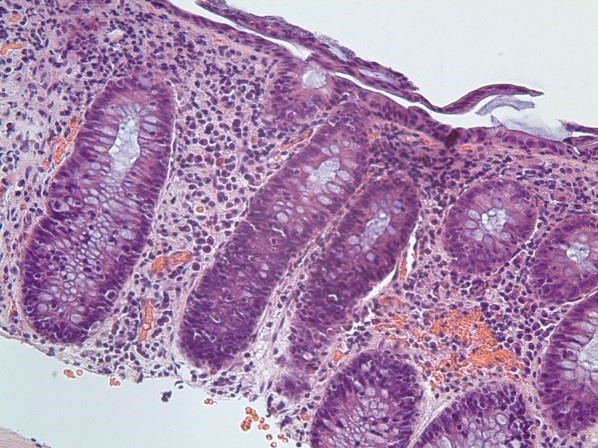
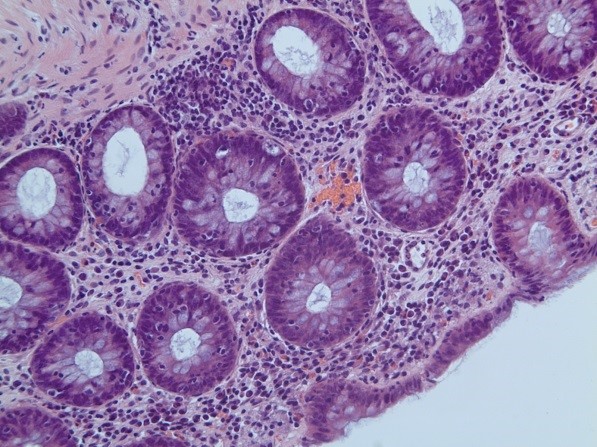
Figure 2: Histology patterns associated with enterocolitis induced by treatment
(A) Crypt abscess with crypt distortion and surrounding plasma cell infiltrate (chronic inflammatory bowel disease pattern; arrow). (B) and (C) showing crypts infiltrated by lymphocytes with surrounding mild chronic inflammation (lymphocytic colitis pattern). All images show haematoxylin and eosin staining viewed at x200 magnification.
Question 2
The patient responded to intravenous methylprednisolone 1 mg/kg and was discharged with a course of oral prednisolone. However, relapse occurred when steroids were weaned and, therefore, he was readmitted and given 5 mg/kg infliximab, with significant improvement within 48 h. He was discharged and prescribed 60 mg oral prednisolone daily with a planned reduction of 10 mg per week. Further intravenous infliximab treatments were prescribed at weeks 2 and 6 of treatment.
Question 3
Unfortunately, the patient remained steroid dependent with symptoms recurring when prednisolone was reduced to below 30 mg. He also developed side effects of proximal myopathy, a cushingoid appearance, low mood, and required an increased dose of insulin to control his blood sugar levels.
Repeat endoscopy demonstrated more severe inflammation in the same distribution. Sigmoid colitis was seen on CT as well as evidence of disease progression within the liver. Symptoms improved but did not completely resolve despite reintroduction of intravenous methylprednisolone for 72 h. Repeat stool cultures remained negative for bacteria and viruses. He was, therefore, started on 300 mg vedolizumab intravenously with further doses planned for 2 and 6 weeks.
Again, there was a good response within 48 h and the patient was discharged with a plan to taper the dose of steroids. After this treatment, they were able to be stopped without further relapse. The patient has since received further chemotherapy (docetaxel) but is not being considered for further immunotherapy.
The patient responded to intravenous methylprednisolone 1 mg/kg and was discharged with a course of oral prednisolone. However, relapse occurred when steroids were weaned and, therefore, he was readmitted and given 5 mg/kg infliximab, with significant improvement within 48 h. He was discharged and prescribed 60 mg oral prednisolone daily with a planned reduction of 10 mg per week. Further intravenous infliximab treatments were prescribed at weeks 2 and 6 of treatment.
Summary
Augmentation of the anti-tumour activity of the immune system with immune-checkpoint-inhibitor therapy is improving survival outcomes in advanced disease in patients with a range of cancer diagnoses (most commonly melanoma, lung cancer, renal cell carcinoma, and lymphoma). However, new therapies have led to novel challenges in managing multi-system adverse effects.
The principles of the BSG-endorsed guidance focus upon diagnosis of all suspected cases by endoscopic and histological evaluation, early initiation of appropriate corticosteroid therapy, and timely escalation to biologic therapies if there is no response to steroids.5 The response rate to infliximab is more than 80%, but vedolizumab has a more gut-specific mode of action and might be used first line in the future. Observational data suggest remission rates similar to those for infliximab.5,6
It is essential for each oncology centre to develop its own pathway with the local gastroenterology team to optimise management for patients with enterocolitis induced by treatment with immune checkpoint inhibitors. An example pathway is shown in Figure 3. These patients are best managed by oncologists with input from gastroenterologists, due to the multi-system nature of the immune-related complications. Good communication and development of expertise are crucial. The long-term immunotherapy plan will be guided by oncologists using the latest guidelines,7 but a guiding principle is for therapy to continue if symptoms are grade 1, pause and restart for grade 2 symptoms, and usually stop for symptoms of grade 3 severity or worse.
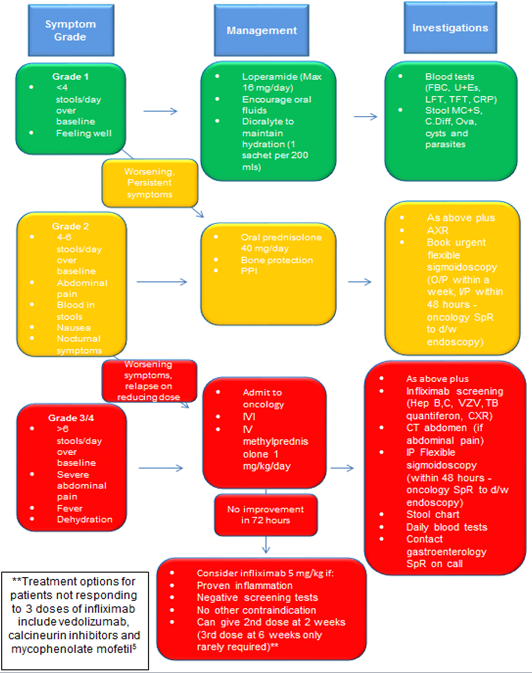
Figure 3: Leeds immunotherapy colitis management ‘traffic light’ pathway
Abbreviations: FBC=full blood count; U+Es=urea and electrolytes; LFTs=liver function tests; TFT=thyroid function tests; CRP=C-reactive protein; MC+S=microscopy, culture, and sensitivity; C. Diff=Clostridium difficile; PPI=proton-pump inhibitor; AXR=abdominal X-ray; O/P=outpatient; I/P=inpatient; SpR=specialist registrar; d/w=discuss with; IVI=intravenous infusion of fluids; IV=intravenous; Hep B, C=hepatitis B and C; VZV=varicella zoster virus; TB=tuberculosis; CXR=chest X-ray; CT=computerised tomography.
Author Biographies

Dr Melanie Cuffe is an internal medicine trainee at Leeds Teaching Hospitals where she aspires to progress beyond her inpatient care experience and develop a career in Gastroenterology.

Dr Nigel Scott is a consultant histopathologist with 25 years’ experience working at the Leeds Teaching Hospitals NHS Trust. He has a particular interest in gastrointestinal pathology, including chronic inflammatory bowel disease, malignant polyps, and colorectal cancer.

Clare Donnellan is a consultant gastroenterologist working at Leeds Teaching Hospitals, where there is a large oncology team. She became interested in gastrointestinal side effects of immunotherapy after looking after a patient who developed colitis in one of the first melanoma trials. She now regularly sees patients with diarrhoea after immunotherapy.
CME
Rheumatological Manifestations of IBD for the Practising Clinician
03 April 2025
IBD Surveillance Colonoscopy – This is how I do it
03 December 2024
SSG Digest This! Simulated multi-disciplinary meeting: IBD scenarios
16 October 2024
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm (accessed March 2, 2022).
- Geukes Foppen MHG, Rozeman EA, van Wilpe S, et al. Immune checkpoint inhibition-related colitis; symptoms, endoscopic features, histology and response to management. ESMO Open 2018;3:e000278.
- Abu-Sbeih H, Ali FS, Luo W, Qiao W, Raju GS, Wang Y. Importance of endoscopic and histological evaluation in the management of immune checkpoint inhibitor-induced colitis. J Immunother Cancer 2018;6:95.
- Wang Y, Abu-Sbeih H, Mao et al. Endoscopic and histologic features of immune checkpoint inhibitor-related colitis. Inflamm Bowel Dis 2018;24:1695-1705.
- Powell N, Ibraheim H, Raine T, et al. British Society of Gastroenterology endorsed guidance for the management of immune checkpoint inhibitor-induced enterocolitis. Lancet Gastroenterol Hepatol 2020;5:680-698
- Zou F, Faleck D, Thomas A, et al. Efficacy and safety of vedolizumab and infliximab treatment for immune-mediated diarrhea and colitis in patients with cancer: a two center observational study. J Immunother Cancer 2021;9(11):
- Haanen J, Carbonnel F, Robert C, et al. Clinical practice guidelines: management of toxicities from immunotherapy. Ann Oncol 2017;28(suppl 4):iv119-142